The objections that had been raised against Arrhenius also had to be faced. Wouldn't the immense volume of the oceans absorb all the extra CO2? Callendar countered that the thin layer of ocean surface waters would quickly saturate, and it would take thousands of years for the rest of the oceans to turn over and be fully exposed to the air. But nobody knew the actual turnover rate, and it seemed that the oceans would have time to handle any extra gases. According to a well-known estimate published in 1924, even without ocean absorption it would take 500 years for fuel combustion to double the amount of CO2 in the atmosphere. Callendar tried to explain that the laboratory spectral measurements were woefully incomplete.
Gathering scattered observational data, he argued that there were parts of the spectrum where the CO2 bands did not overlap with water vapor absorption. Some scientists found this convincing, or at least kept an open mind on the question. But it remained the standard view that, as an official U.S. Weather Bureau publication put it, the masking of CO2 absorption by water vapor was a "fatal blow" to the CO2 theory. Therefore, said this authority, "no probable increase in atmospheric CO2 could materially affect" the balance of radiation. Most damaging of all, Callendar's calculations of the greenhouse effect temperature rise, like Arrhenius's, ignored much of the real world's physics.
Worse, any rise in temperature would allow the air to hold more moisture, which would probably mean more clouds that would reflect sunlight and thus preserve the natural balance. Callendar admitted that the actual climate change would depend on interactions involving changes of cloud cover and other processes that no scientist of the time could reliably calculate. Few thought it worthwhile to speculate about such dubious questions, where data were rudimentary and theory was no more than hand-waving. Better to rest with the widespread conviction that the atmosphere was a stable, automatically self-regulated system. The notion that humanity could permanently change global climate was implausible on the face of it, hardly worth a scientist's attention. The scientists who brushed aside Callendar's claims were reasoning well enough.
Research by definition is done at the frontier of ignorance. Like nearly everyone described in these essays, Callendar had to use intuition as well as logic to draw any conclusions at all from the murky data and theories at his disposal. Like nearly everyone, he argued for conclusions that mingled the true with the false, leaving it to later workers to peel away the bad parts.
While he could not prove that greenhouse effect warming was underway, he had given sound reasons to reconsider the question. His claims rescued the idea of global warming from obscurity and thrust it into the marketplace of scientific ideas. Eventually, these individual laws were combined into a single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures. We will consider the key developments in individual relationships , then put them together in the ideal gas law. It was reassuring that there seemed scant possibility of a Venus-style runaway greenhouse apocalypse. The Earth had been virtually a different planet, with tropical forests near the poles and sea levels a hundred meters higher.
To be sure, it would take many thousands of years to melt entire polar ice caps. But in the meantime even a modest sea-level rise would disrupt humanity's teeming coastal populations. For the real planet, a rise in temperature had evidently not been limited by increased cloud reflection or the like.
The rise had instead apparently been amplified by positive feedbacks, as ice and oceans and vegetation responded over centuries to the changing conditions with darker surfaces and their own gas emissions. The computer models did not take these slow feedback loops into account. Hansen and others argued that humanity risked setting off a chain reaction that would eventually bring an altogether catastrophic planetary change.
As sometimes happens with landmark scientific papers, written in haste while understanding just begins to dawn, Revelle's explanation was hard to grasp. Other scientists failed to see the point that was obscurely buried in the calculations, and continued to deny there was a greenhouse effect problem. They explained the seawater buffering clearly — so clearly that during the next few years, some scientists cited Bolin and Eriksson's paper for this decisive insight rather than Revelle and Suess's . The central insight was that although seawater did rapidly absorb CO2, most of the added gas would promptly evaporate back into the air before the slow oceanic circulation swept it into the abyss. To be sure, the chemistry of air and seawater would eventually reach an equilibrium — but that could take thousands of years.
Arrhenius had not concerned himself with timescales shorter than that, but geoscientists in the 1950s did. Keeling's Curve TOP OF PAGE In the late 1950s a few American scientists, starting with Plass, tentatively began to inform the public that greenhouse gases might become a problem within the foreseeable future. Revelle in particular warned journalists and government officials that greenhouse warming deserved serious attention. The stakes were revealed when Bolin and Eriksson pursued the consequences of their calculation to the end. They assumed industrial production would climb exponentially, and figured that atmospheric CO2 would rise some 25% by the year 2000.
That was a far swifter rise than anyone before had suggested. As the New York Times reported in a brief note, Bolin suggested that the effect on climate "might be radical." In 1962, a still stronger warning was sounded by the Russian climate expert Mikhail Budyko. His calculations of the exponential growth of industrial civilization suggested a drastic global warming within the next century or so. Ice cores have been drilled in ice sheets worldwide, but notably in Greenland and Antarctica. High rates of snow accumulation provide excellent time resolution, and bubbles in the ice core preserve actual samples of the world's ancient atmosphere.
Through analysis of ice cores, scientists learn about glacial-interglacial cycles, changing atmospheric carbon dioxide levels, and climate stability over the last 10,000 years. A major stimulus was a controversy that erupted in the early 1970s and stubbornly resisted resolution. National economic statistics yielded reliable figures for how much CO2 humanity put into the air each year from burning fossil fuels. The measurements of the annual increase by Keeling and others showed that less than half of the new carbon could be found in the atmosphere. Oceanographers calculated how much of the gas the oceans took up, while other scientists calculated how much the biosphere took up or emitted.
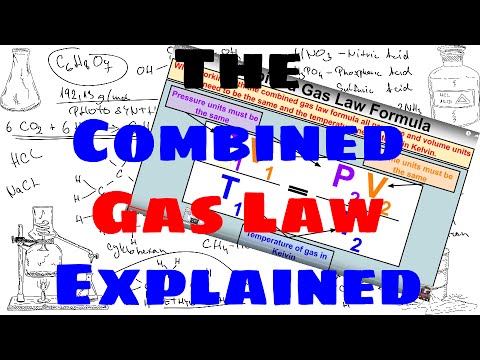
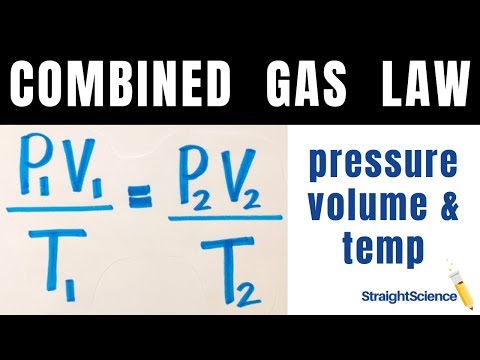
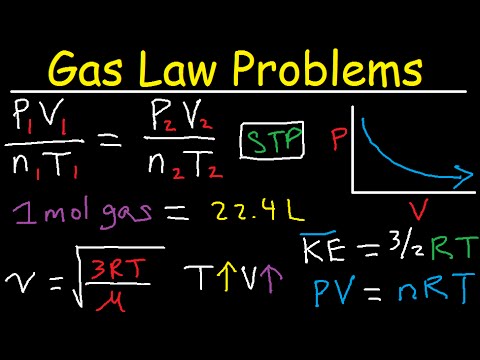
The numbers didn't add up — some of the carbon was "missing." Plainly, scientists did not understand important parts of the carbon cycle. Looking at large-scale climate changes, such as between ice ages and warm periods, they turned up a variety of possible interactions with climate involving plant life and ocean chemistry. The papers addressing these topics became increasingly complex. And is a proportionality constant that relates the values of pressure, volume, amount, and temperature of a gas sample.
The variables in this equation do not have the subscripts i and f to indicate an initial condition and a final condition. The ideal gas law relates the four independent properties of a gas under any conditions. Gases whose properties of P, V, and T are accurately described by the ideal gas law are said to exhibit ideal behavior or to approximate the traits of an ideal gas. An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter.
Although all the calculations presented in this module assume ideal behavior, this assumption is only reasonable for gases under conditions of relatively low pressure and high temperature. In the final module of this chapter, a modified gas law will be introduced that accounts for the non-ideal behavior observed for many gases at relatively high pressures and low temperatures. There were many ways temperature or other climate features could influence the carbon dioxide level one way or another.
Perhaps variations of temperature and of weather patterns caused land vegetation to release extra CO2, or take it up... Perhaps the oceans were involved through massive changes in their circulation or ice cover... Or perhaps there were still more complicated and obscure effects. Into the 21st century, scientists kept finding new ways that warming would push more of the gas into the atmosphere.
How Do You Find Volume Of A Gas As one of them remarked, "it is difficult to explain the demise of the ice sheets without the added heating from CO2 ... this gas has killed ice sheets in the past and may do so again." The new ice cores suggested that a powerful feedback amplified the changes in sunlight. The crucial fact was that a slight warming would cause the level of greenhouse gases to rise slightly. For one thing, warmer oceans would evaporate out more gas.
For another, as the vast Arctic tundras warmed up, the bogs would emit more CO2 . The greenhouse effect of these gases would raise the temperature a little more, which would cause more emission of gases, which would... And so forth, hauling the planet step by step into a warm period. Many thousands of years later, the process would reverse when the sunlight falling in key latitudes weakened. Bogs and oceans would absorb greenhouse gases, ice would build up, and the planet would slide back into an ice age.
This finally explained how tiny shifts in the Earth's orbit could set the timing of the enormous swings of glacial cycles. Some scientists took up the old argument that fertilization of plant life by additional CO2, together with uptake by the oceans, would keep the level of gas from rising too sharply. Keeling, however, warned that by the middle of the next century, plants could well reach their limit in taking up carbon . Further, there would eventually be so much CO2 in the ocean surface waters that the oceans would not be able to absorb additional gas as rapidly as at present. Keeling kept refining and improving his measurements of the CO2 level in the atmosphere to extract more information.
The curve did not climb smoothly, but stuttered through a seasonal cycle, plus mysterious spells of faster and slower growth. But over a long term, say a decade, the rise was clearly as inexorable as the tides. Meanwhile, computer models were coming into better agreement on the future warming to be expected from increased CO2. It was getting increasingly difficult for scientists to believe that the greenhouse effect was no cause for worry.
But would adding carbon dioxide in the upper layers of the air significantly change the surface temperature? Only detailed computations, point by point across the infrared spectrum and layer by layer down through the atmosphere, could answer that question. By 1956, such computations could be carried out thanks to the increasing power of digital computers. The physicist Gilbert N. Plass took up the challenge of calculating the transmission of radiation through the atmosphere . He nailed down the likelihood that adding more CO2 would increase the interference with infrared radiation.
Going beyond this qualitative result, Plass calculated that doubling the level would bring a 3-4°C rise. Assuming that emissions would continue at the current rate, he expected that human activity would raise the average global temperature "at the rate of 1.1 degree C per century." Boyle's law is named after Robert Boyle, who first stated it in 1662. Increasing the amount of space available will allow the gas particles to spread farther apart, but this reduces the number of particles available to collide with the container, so pressure decreases. Decreasing the volume of the container forces the particles to collide more often, so pressure is increased. As more air goes in, the gas molecules get packed together, reducing their volume.
As long as the temperature stays the same, the pressure increases. The relationship between pressure and volume of a gas, under conditions of constant temperature, is inversely proportional — if pressure increases, volume decreases. Either pressure or volume can change, and the other factor responds accordingly, changing in the opposite direction by a proportional amount.
That is, if the volume is cut in half, the pressure doubles. This inversely proportional relationship between pressure and volume, under constant temperature, is called Boyle's Law. This relationship between temperature and pressure is observed for any sample of gas confined to a constant volume. An example of experimental pressure-temperature data is shown for a sample of air under these conditions in Figure 9.11. An example of experimental pressure-temperature data is shown for a sample of air under these conditions in Figure 3.
If we had not emitted so much carbon, we would be on our way back to an ice age in a few thousand years. However, we have increased atmospheric CO2 by about 40% since the Industrial Revolution of the 1800's. The added heat trapped by these and other greenhouse gases will now combine with the natural changes in solar forcing from orbital changes. I don't know the exact result of this new energy balance, but it is safe to say we can expect a significantly modified future.
I believe global temperature change from an ice age to a warm period has tended to be about 3-5ºC. Since 1850, we have measured a global average increase in temperature that is almost 1ºC and rising. International efforts such as the Paris Climate Agreement are trying to limit human-caused warming to 2ºC. That might give you a sense of our impact–in just ~200 years–relative to the natural fluctuations we've seen in the past which occur over 1000s to 10,000s of years.
They addressed the gas as simply one component in their study of biological, oceanographic or meteorological systems. Most stuck with the old assumption that the Earth's geochemistry was dominated by stable mineral processes, operating on a planetary scale over millions of years. People did not easily grasp how sensitive the Earth's atmosphere was to biological forces — the totality of the planet's living activity — to say nothing of the fraction of that activity affected by humanity.
Yet the theory that atmospheric CO2 variations could change the climate was never altogether forgotten. An idea so simple on the face of it, an idea advanced by outstanding figures like Arrhenius and Chamberlin, had to be mentioned in textbooks and review articles if only to refute it. Arrhenius's outmoded hypothesis persisted in a ghostly afterlife. Callendar's Advocacy TOP OF PAGE The greenhouse warming theory found a lone advocate. In 1938 an English engineer, Guy Stewart Callendar, tried to revive the old idea. An expert on steam technology, Callendar apparently took up meteorology as a hobby to fill his spare time.
Many people, looking at weather stories from the past, had been saying that a warming trend was underway. When Callendar compiled measurements of temperatures from the 19th century on, he found they were right. He went on to dig up and evaluate old measurements of atmospheric CO2 concentrations. He concluded that over the past hundred years the concentration of the gas had increased by about 10%. This rise, Callendar asserted, could explain the observed warming.
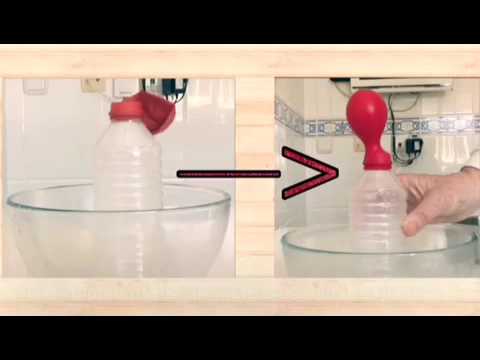
Earth's atmosphere is not like the air inside a sealed wine bottle. Gas molecules want to move, and they will expand to fill the volume within which they are contained. Confined to a tightly sealed container such as a corked wine bottle at constant temperature of about degrees F, gasses have no room or enough "excitement" to expand and move around. They settle into layers based mostly on their molecular weights. However, the Earth's atmosphere is much more expansive than a wine bottle. CO2 does not break down until about 80 kilometers from the Earth's surface, giving atmospheric gases a huge expanse to occupy.
Excited by the heat radiating from the Sun into the atmosphere, molecules move rapidly. As they bang into each other , the gas molecules intermingle, rather than settling in stratified layers. The density of a gas increases as temperatures get colder. So, because temperatures decrease as we reach higher altitudes, gases become denser at higher altitudes.





















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.